Providing a minimally invasive means to permanently treat sinus obstructions
Sinus conditions, such as chronic inflammation and obstructed airways, have traditionally been treated with surgery. Sections of eggshell-like bone would be cut out of the patient’s sinuses and removed to increase airflow in breathing. Traditional methods were painful, resulted in frequent complications and required the use of drug adjuncts to reduce bleeding and pain. Acclarent (Johnson & Johnson’s ENT company) pioneered the use of an expanding balloon that, when inflated with saline, would permanently dilate the airway in question. This created an outpatient procedure with dramatically reduced complication rates with limited drug adjuncts and no anesthesia. The technology was sound, the means of accurately and safely inflating the balloons in the sinus was less so. Since the advent of Balloon Sinuplasty, the means of inflating the balloons was problematic. There are multiple steps to inflation. Users are required to generate high forces, hold position and determine time and pressure. All inflators on the market were perceived as cheap, inaccurate and clumsy to use. The SSID project changed that. Striving for cost equivalency, increased accuracy, and a dramatically improved customer experience, the SSID (later called SE) was released, becoming the first user-friendly, instinctually operated sinuplasty balloon inflator on the market.
A minimally invasive means of treating chronic sinus and airway conditions.
Crude, clumsy and difficult to use, existing inflators offered little to accommodate the users.
SSID was a relatively quick project, requiring rapid conceptual development and testing and execution.
Final 3 conceptual approaches
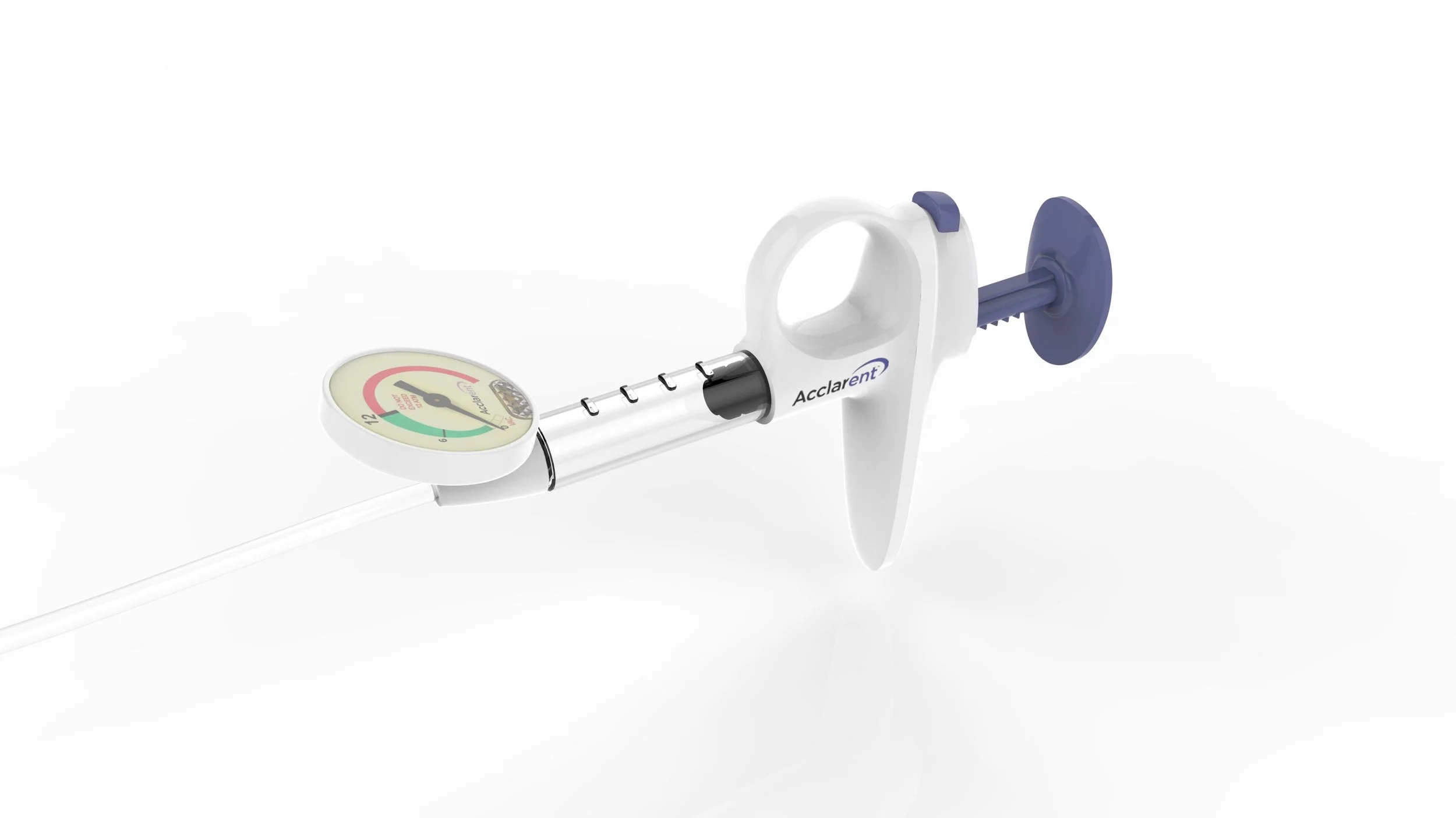
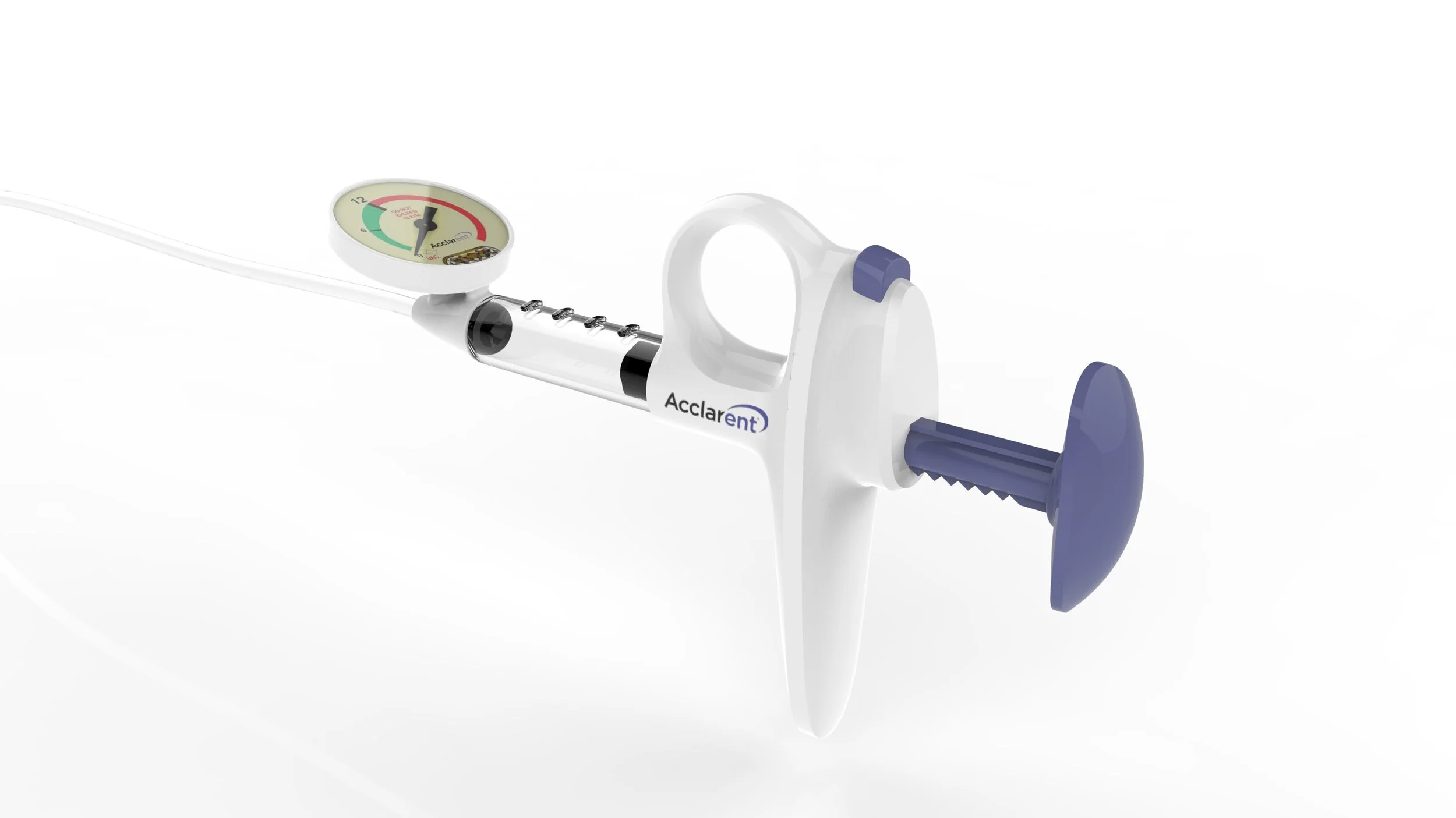
Final production design
The SSID (SE)
The SSID device was successfully launched to very positive reviews and Acclarent continues to be the leader in minimally invasive ENT procedures.
Click on images below for expanded viewing of SSID